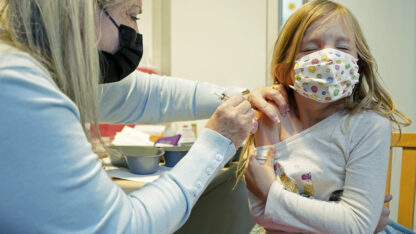
COVID-19 vaccines are rolling out to Georgia's young children
The latest round of COVID-19 shots is rolling out and Georgia health officials are encouraging all families to get their children ages 6 months and up vaccinated.
“I know many parents with very young children have been anticipating this day. We now know, based on rigorous scientific review, that the vaccines available here in the United States can be used safely and effectively in children under five,” Atlanta-based Centers for Disease Control and Prevention director Dr. Rochelle Walensky told the New York Times.
“Parents, I strongly encourage you to get your children vaccinated. If you have questions, talk to your child’s [healthcare] provider to learn more about the benefits of this vaccine.”
The CDC greenlit the Pfizer and Moderna COVID-19 vaccines for kids under 5 over the weekend following their emergency use authorization at the U.S. Food and Drug Administration.
The Georgia Department of Health reports that at least 225 medical providers across the state, including pediatricians, family practice doctors, health departments and independent pharmacies, pre-ordered the new vaccines in preparation for their authorization for young children.
A spokesperson for the department says the providers have ordered 39,400 doses of the Pfizer-BioNTech vaccine and another 14,000 doses of the Moderna vaccine.
“Together, with science leading the charge, we have taken another important step forward in our nation’s fight against COVID-19. We know millions of parents and caregivers are eager to get their young children vaccinated, and with today’s decision, they can. I encourage parents and caregivers with questions to talk to their doctor, nurse, or local pharmacist to learn more about the benefits of vaccinations and the importance of protecting their children by getting them vaccinated,” Walensky said.
From the FDA:
The Moderna COVID-19 Vaccine is administered as a primary series of two doses, one month apart, to individuals 6 months through 17 years of age. The vaccine is also authorized to provide a third primary series dose at least one month following the second dose for individuals in this age group who have been determined to have certain kinds of immunocompromise.
The Pfizer-BioNTech COVID-19 Vaccine is administered as a primary series of three doses in which the initial two doses are administered three weeks apart followed by a third dose administered at least eight weeks after the second dose in individuals 6 months through 4 years of age.
Information about each vaccine is available in the fact sheets for healthcare providers administering vaccine and the fact sheets for recipients and caregivers.