A $14K Annual HIV Prevention Pill Just Became A Lot Cheaper; Georgians May See Increased Access
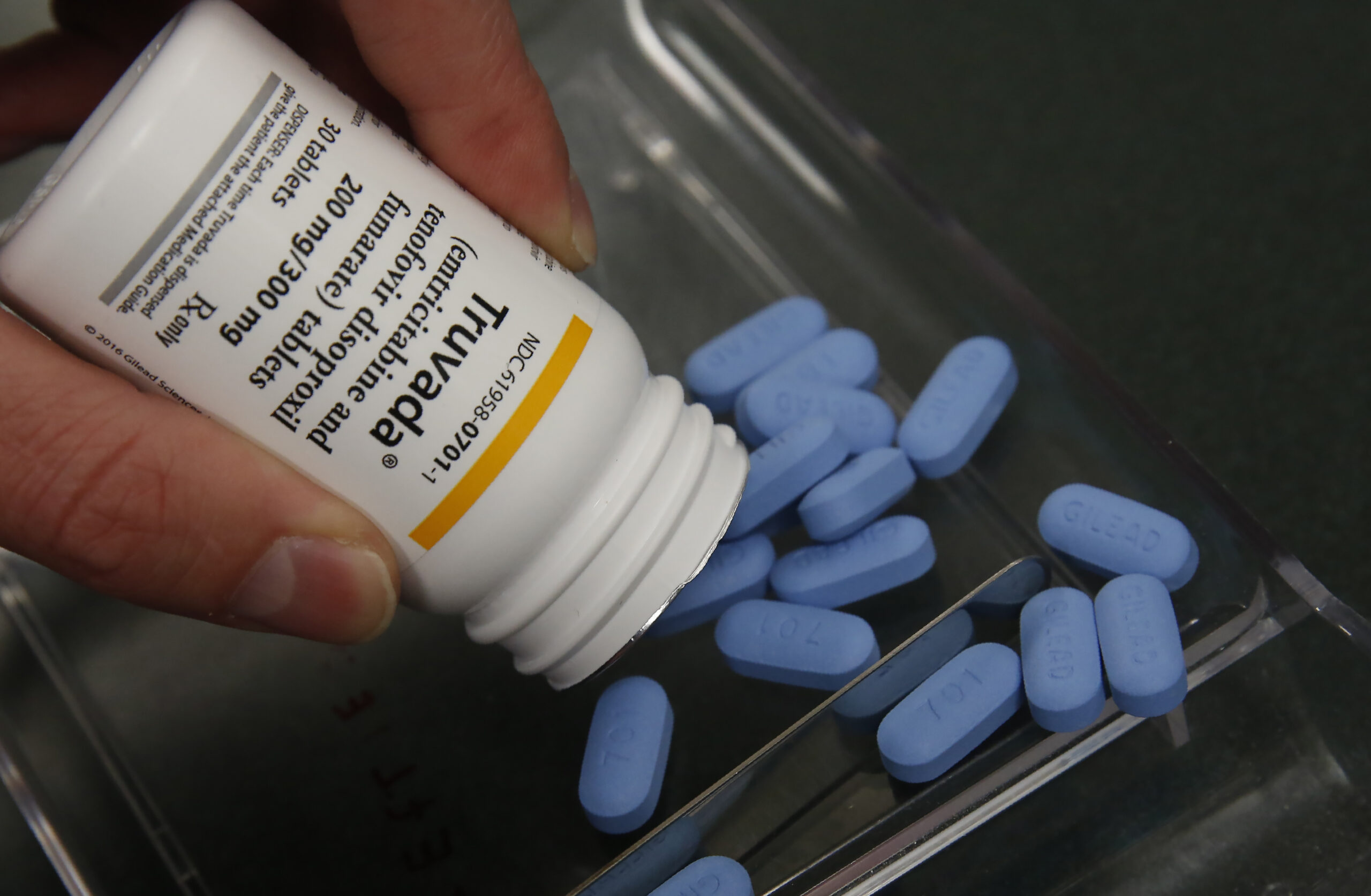
Truvada, the brand name of the drug approved in 2012 for widespread PrEP use, carried a list price of more than $14,000 a year. Now, Truvada is going off-patent, which means it could soon become much cheaper.
Rich Pedroncelli / Associated Press file
Georgia — and specifically Atlanta — has long been among the top areas for new HIV diagnoses.
The same city that headquarters the Centers for Disease Control and Prevention still has a long way to go in fighting the epidemic, according to Emory Center for AIDS Research co-director Carlos del Rio.
A 2018 Emory Health Digest article found poverty, lack of insurance and stigma have all combined to make HIV the leading cause of death among Black men ages 35 to 44 in Georgia.
And it’s not just Georgia.
Half of all new cases in the U.S. in 2018 were in the South, and nearly 75% of those cases were among Black and Hispanic people, according to the CDC.
Public health officials say the most-promising resource over the last decade has been a pill used as a “Pre-exposure prophylaxis,” or PrEP.
Those who are HIV-negative but at risk of contracting HIV can take PrEP to avoid getting the virus. Truvada, the brand name of the drug approved in 2012 for widespread PrEP use, carried a list price of more than $14,000 a year. Negotiations with insurance companies often made it cheaper.
Now, Truvada is going off-patent, which means it could soon become much cheaper.
Dan Gorenstein hosts the podcast, Tradeoffs, and Ryan Levi is its producer. The two spent months digging into whether cheaper PrEP will finally increase access — and reduce infection rates — especially in Georgia and across the South.
Lily Oppenheimer contributed to this report.








